On average, patients with chronic illnesses follow their prescribed treatments about 50 percent of the time. That’s a problem. If drugs aren’t taken regularly, on time, and in the right doses, the treatment may not work, and the person’s condition can worsen.
The issue isn’t that people are unwilling to take their prescriptions. It’s that some drugs, like HIV medications, require unwavering commitment. And essential medicines, like insulin, can be brutally expensive. Plus, the Covid pandemic illustrated the difficulties of delivering perishable follow-up vaccine shots to regions with no cold chain. “Are we really squeezing all the utility out of those drugs and vaccines?” asks Kevin McHugh, a bioengineer at Rice University. “The answer is, in general, no. And sometimes we’re missing out on a lot.”
For example, the injectable drug bevacizumab can be used to treat macular degeneration, a leading cause of blindness. But even though it’s effective, dosing adherence is notoriously low. “People hate getting injections into their eyes,” McHugh says. “And I don’t blame them at all—that’s terrible.”

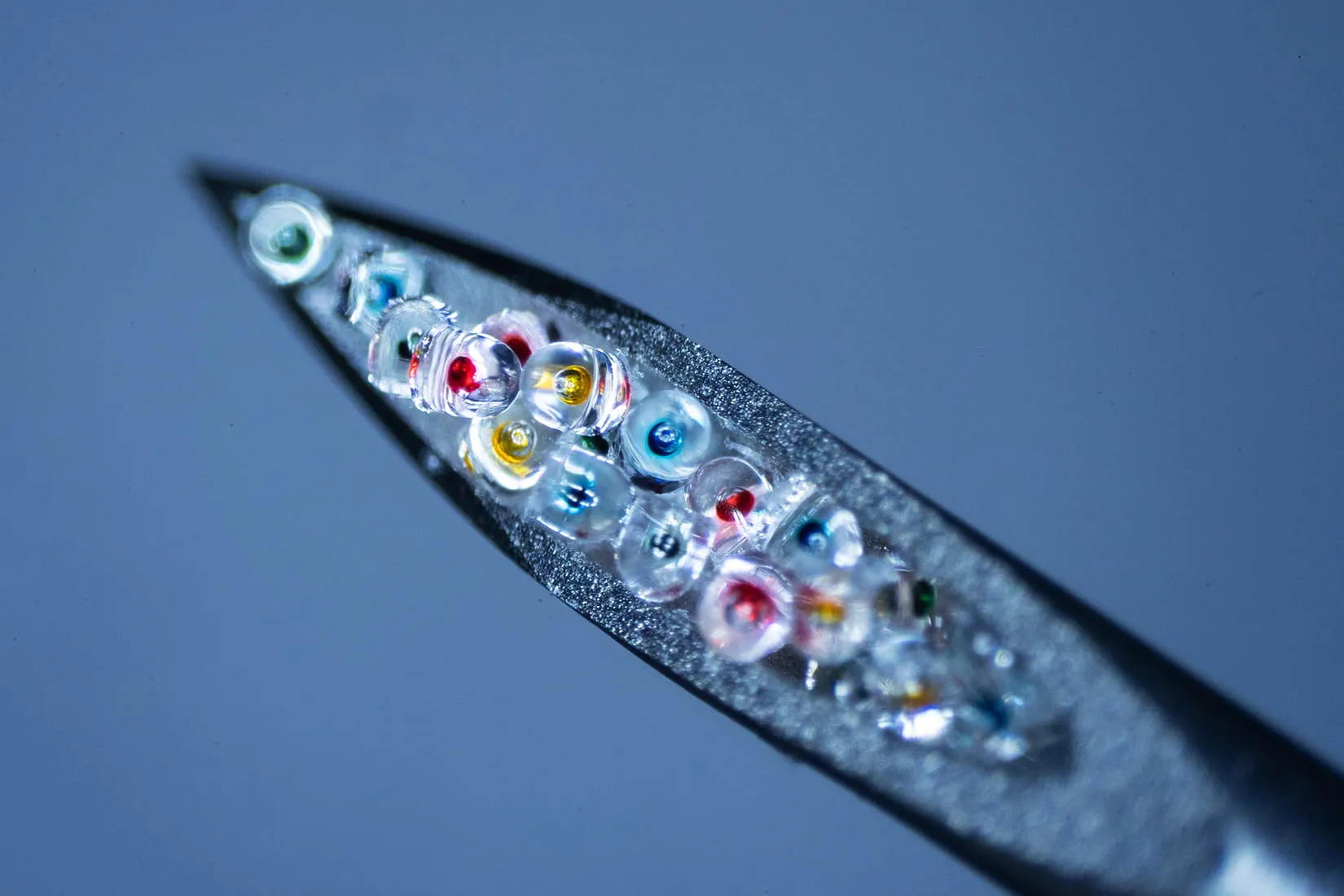
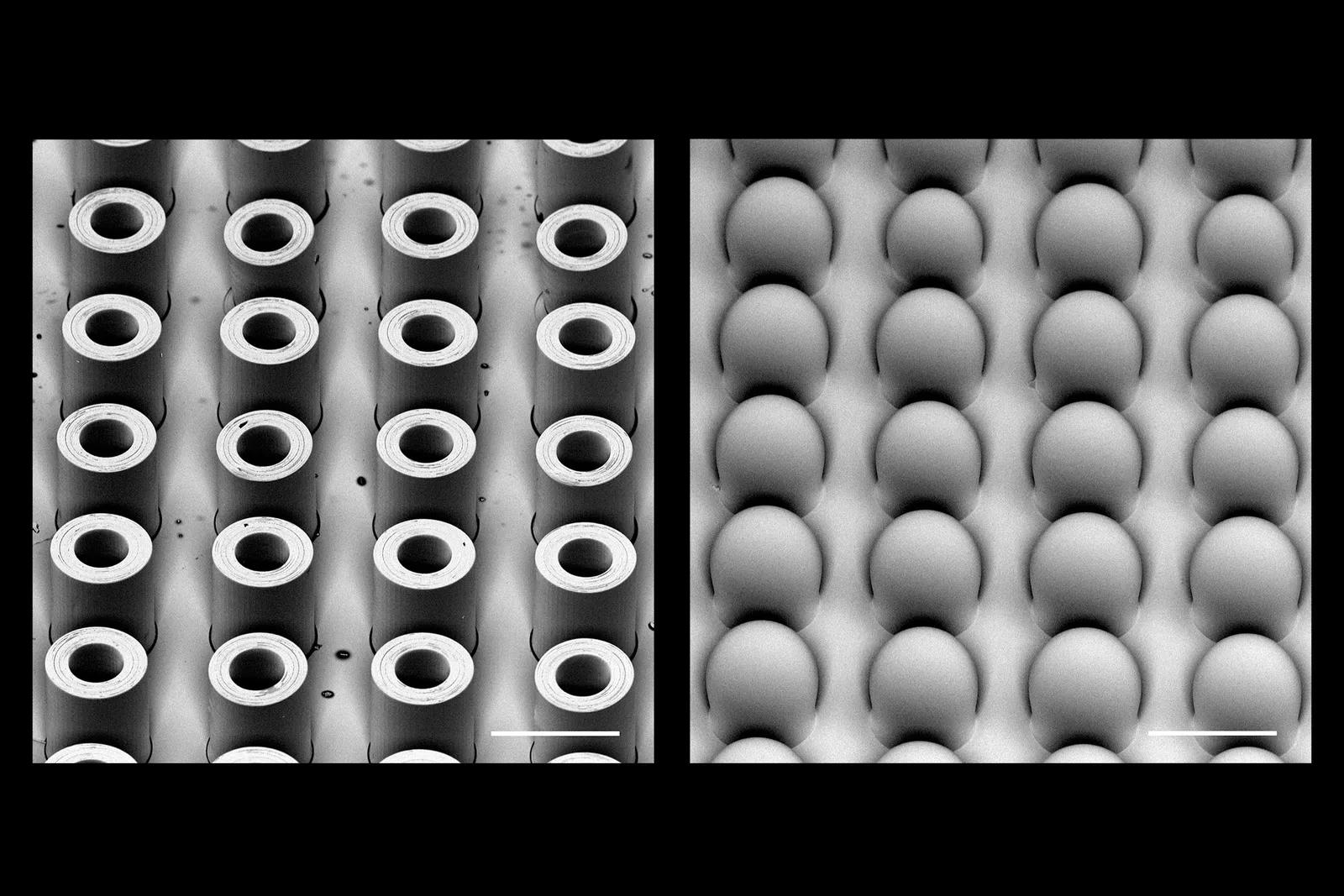
In the June issue of Advanced Materials, McHugh’s team described how their system works. It starts with an injection containing hundreds of tiny microplastic particles, each encapsulating a small dose of a drug. These minuscule capsules are made of the polymer PLGA, which our bodies break down safely. By adjusting the molecular weight of the polymer used for each capsule, the scientists can control how fast they erode and release medication. In this study, the team demonstrated a single shot containing four groups of microparticles that released their contents at 10, 15, 17, and 36 days after injection.

 Loading comments...
Loading comments...
